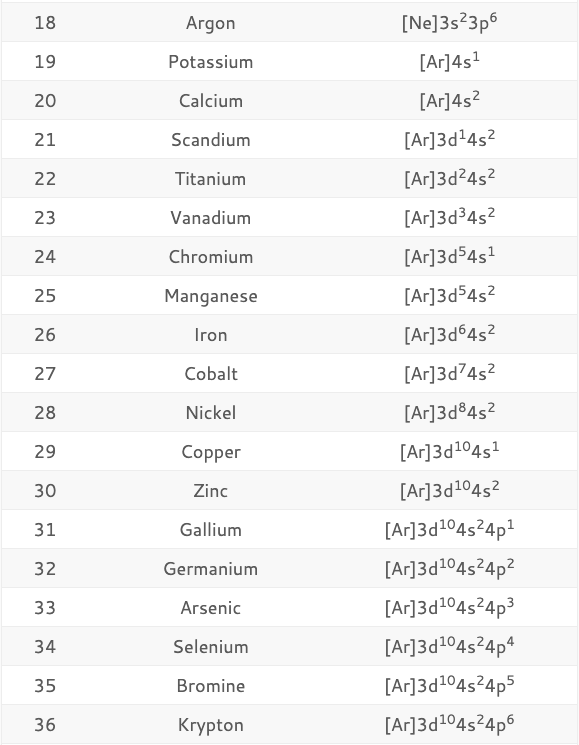
Valence Electron Configuration Chart . But the valency of elements, when combined with h or o first, increases from 1 to 4 and then it reduces to zero. 16 s [ne] 3s 2 3p 4:
Electron Configuration Chart Of All Elements from periodictable.me
So based on what we know about the quantum numbers and using the chart. Valence electrons h 1s1 1s1 1 li 1s2 2s1 2s1 1 na 1s2 2s2 2p6 3s1 3s1 1 h, li, and na are all in group 1 (or 1a) on the periodic chart. The valence electron configuration for selenium is 4 s 2 4 p 4.
Electron Configuration Chart Of All Elements
5 b 1s 2 2s 2 2p 1: The ionization energy is the amount of energy required to remove a valence electron. We allow this kind of valence shell electron configuration graphic could possibly be the most trending subject next we portion it in google lead or facebook. “electrons in the outer shells that are not filled are called valence electrons”.
Source: acemichael888.weebly.com
The valence electron configuration for selenium is 4s 2 4p 4. Prep after school/summer school parental involvement professional development. The closer an electron to the nucleus, the greater the ionization energy since the attraction between the negative electron and positive nucleus is. 13 al [ne] 3s 2 3p 1: While moving left to right across a period, the number of.
Source: www.youtube.com
“electrons in the outer shells that are not filled are called valence electrons”. Silicon [ne]3s 2 3p 2: Aluminum [ne]3s 2 3p 1: Valence is the number of electrons an atom must lose or gain to attain the nearest noble gas or inert gas electronic configuration. 8 o 1s 2 2s 2 2p 4:
Source: sciencetrends.com
Configuration definition, the relative disposition or arrangement of the parts or elements of a thing. 11 na [ne] 3s 1: The outermost shell is already fulfilled. 9 f 1s 2 2s 2 2p 5: 12 mg [ne] 3s 2:
Source: periodictable.me
Element electron configuration end config. Electron configurations in the periodic table. 5 b 1s 2 2s 2 2p 1: The valence electron configuration for aluminum is 3s 2 3p 1. Write the element’s chemical symbol 2.
Source: chem.libretexts.org
Valence electrons in neon (ne). 12 mg [ne] 3s 2: It will lose 1 electron to complete its octet. 119 rows valence electrons in fluorine (f) 7: 9 f 1s 2 2s 2 2p 5:
Source: www.webelements.com
4 be 1s 2 2s 2: Look on the periodic table to see what group the element is in 3. For example, the electronic configuration of phosphorus (p) is 1s 2 2s 2 2p 6 3s 2 3p 3 so that there are 5 valence electrons (3s 2 3p 3), corresponding to a maximum valence for p of 5 as.
Source: www.carolina.com
16 s [ne] 3s 2 3p 4: Dont you come here to know some further unique pot de fleurs pas cher. 8 o 1s 2 2s 2 2p 4: Fluorine [he]2s 2 2p 5: 6 c 1s 2 2s 2 2p 2:
Source: socratic.org
12 mg [ne] 3s 2: 119 rows valence electrons in fluorine (f) 7: In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration to the periodic properties of the elements. Neon [he]2s 2 2p 6: 1 h 1s 1 :
Source: chem.libretexts.org
Steps for drawing an electron dot diagram 1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: Here are the configurations of be, mg, and ca 13) state which of the first,. 11 na [ne] 3s 1:
Source: courses.lumenlearning.com
In writing the electron configuration for beryllium the first two electrons will go in the 1s orbital. Valence electron configuration chart learning objectives to correlate the arrangement of atoms in the periodic table results in blocks corresponding to filling of the ns, np, nd, and nf orbitals as you have learned, the electron configurations of the elements explain the otherwise.
Source: www.sparknotes.com
Dont you come here to know some further unique pot de fleurs pas cher. Shop by product curriculum mastery games flip charts visual. While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. 16 s [ne] 3s 2 3p 4: Look on the periodic table to see what.
Source: en.wikibooks.org
9 f 1s 2 2s 2 2p 5: Draw the dots around the chemical symbol starting at the top and moving clockwise around the symbol But the valency of elements, when combined with h or o first, increases from 1 to 4 and then it reduces to zero. Predict where the 11th electron representing the element sodium will be placed..
Source: periodictable.me
Predict where the 11th electron representing the element sodium will be placed. Neon [he]2s 2 2p 6: While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. The electron configuration can be determined from where the atom is located in the periodic table and by using the spdf.
Source: www.youtube.com
The content that follows is the substance of general chemistry lecture 26. Dont you come here to know some further unique pot de fleurs pas cher. Nitrogen [he]2s 2 2p 3: Aluminum [ne]3s 2 3p 1: It will lose 1 electron to complete its octet.
Source: terpconnect.umd.edu
We try to introduced in this posting back this may be one of astounding quotation for any valence shell electron configuration options. 1 h 1s 1 : Nitrogen [he]2s 2 2p 3: The closer an electron to the nucleus, the greater the ionization energy since the attraction between the negative electron and positive nucleus is. Steps for drawing an electron.
Source: chemistry.stackexchange.com
Variation of oxidation state along a period. 15 p [ne] 3s 2 3p 3: The outermost shell is already fulfilled. Use the chart in your notes to determine how many valence electrons the element has 4. In this lecture we continue the discussion of quantum numbers and their use in electron configurations as well as the relationship of electron configuration.
Source: mahara.ats2020.eu
The valence electron configuration for selenium is 4 s 2 4 p 4. Dont you come here to know some further unique pot de fleurs pas cher. 7 n 1s 2 2s 2 2p 3: In viewing the pes chart of an element, you are also able to distinguish the different orbital levels and determine the electron configuration. So it.
Source: www.cliffsnotes.com
In viewing the pes chart of an element, you are also able to distinguish the different orbital levels and determine the electron configuration. Since 1s can only hold two electrons the remaining 2 electrons for be go in the 2s orbital. The valence electron configuration for selenium is 4 s 2 4 p 4. Steps for drawing an electron dot.
Source: www.cliffsnotes.com
Today through the use of our article, the users are going to know about certain terms of chemistry and without which the users or anyone cannot master the subject. 9 f 1s 2 2s 2 2p 5: 7 n 1s 2 2s 2 2p 3: Prep after school/summer school parental involvement professional development. The closer an electron to the nucleus,.
Source: en.wikipedia.org
Nitrogen [he]2s 2 2p 3: 119 rows shorthand electron configuration full electron configuration electron. But the valency of elements, when combined with h or o first, increases from 1 to 4 and then it reduces to zero. We try to introduced in this posting back this may be one of astounding quotation for any valence shell electron configuration options. Here.